HIV-associated nephropathy (HIVAN) is frequently observed in HIV/AIDS patients and results in end-stage renal impairment (ESRD). The diagnosis cannot be confirmed by imaging and must be confirmed by renal biopsy.
What is the AIDS virus?
A disorder of the immune system caused by HIV infection. HIV damages the immune system’s CD4 T lymphocytes (CD4 cells), leaving the body susceptible to life-threatening infections and malignancies. The most advanced stage of HIV infection is acquired immunodeficiency syndrome (AIDS). A person with HIV must have an AIDS-defining disease or a CD4 count of less than 200 cells/mm3 to be diagnosed with AIDS (regardless of whether the person has an AIDS-defining condition).
Pathogenesis
HIV infection is the cause of HIVAN, with experimental evidence supporting the hypothesis that direct infection of kidney epithelial cells is sufficient to cause illness. Patients with a risk allele variation of APOL1 that is prevalent in African Americans had a significantly elevated prevalence of HIVAN.
Several HIV-1 genes’ functions in HIVAN pathogenesis have been investigated. Although HIV-1 nef appears to be a key modulator of podocyte proliferation in HIVAN, it is probable that additional HIV-1 genes also contribute to the development of renal impairment. Despite the evidence that genetic variables contribute to vulnerability to HIVAN in humans and animal models, these factors remain largely unknown. Multiple cellular genes, including as mediators of proliferation, apoptosis, inflammation, cell-cell and cell-matrix interactions, have been implicated in the pathogenesis of HIVAN. In the two decades since the initial description of HIVAN, much has been learnt about the mechanisms that contribute to the progression of renal impairment. However, the recent remarkable reductions in HIV/AIDS patient mortality have not been accompanied by a drop in the incidence of ESRD attributable to HIVAN. Therefore, additional study is urgently required to logically devise innovative techniques for the prevention and treatment of this illness.
Clinical features
In 1984, New York City physicians reported a number of individuals with severe AIDS with quickly advancing glomerular disease. In this initial report, all of the affected patients were African Americans or Haitian immigrants; nevertheless, the significance of race was not completely understood until several years later. The typical clinical picture of HIVAN was marked by quickly progressing renal failure, mild to nephrotic range proteinuria, bland urine sediment, and big, highly echogenic kidneys as detected by ultrasonography. In 1999, “AIDS nephropathy” ranked as the third largest cause of end-stage renal disease (ESRD) among African Americans aged 20 to 64.
Diagnosis
- Biopsy is the diagnostic gold standard. Other indicators (such as proteinuria, CD4, and VL) lack specificity.
- Proteinuria, bland urinalysis; nephritic profile suggests another diagnosis.
- Urgent renal Biopsy indicated substantial proteinuria (>1g/24h), rising proteinuria, decreased GFR, or unexplained acute or subacute renal failure.
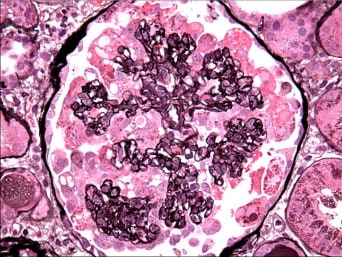
- Pathognomonic characteristics: FSGS, collapsing form; interstitial inflammation +/- fibrosis; microcystic tubular dilatation; tubuloreticular inclusions on electron microscopy (EM)
- Renal ultrasound: echogenic kidneys ranging in size from normal to enlarged.
- Without Biopsy, the following diagnoses are possible: primary FSGS, immune complex glomerulonephritis (GN), hepatitis B or C-associated GN, drug-induced interstitial nephritis, amyloidosis, IgA nephropathy, injection drug use nephrotoxicity, thrombotic microangiopathy, and acute tubular necrosis.
Evaluation of kidney function
- Measure eGFR during ART commencement or change, and at least twice per year in HIV+ patients who are stable. Patients of African descent with CD4 200, high VL, diabetes, hypertension, hepatitis B or C, or familial history with a renal disease history are at a higher risk for kidney injury. Additional risk factors include a history of cocaine or tobacco use, as well as hyperlipidemia.
Assessment of kidney damage:
- Urinalysis or quantitative albuminuria/proteinuria measurement at baseline, ART initiation or change, and at least annually in HIV+ patients.
- Proteinuria 1+ on dipstick, clinically significant albuminuria >300 mg/day (nondiabetics) OR >30 mg/day (diabetics), GFR decline >25% from baseline, or GFR 60ml/min/1.73m2 need renal ultrasonography, early referral to nephrologist, and renal biopsies.
Treatment
No conventional treatment for HIVAN has been discovered, but there is evidence that effective antiretroviral therapy slows the course of the disease. Angiotensin-converting enzyme inhibitors are occasionally prescribed to HIVAN patients, although their efficacy has not been assessed in controlled research. In some circumstances, it has been claimed that steroids are beneficial, although the justification for their use in diseases that are not immune complex-mediated is unclear.
The effects of strong antiretroviral medication on HIVAN have not yet been explored in a controlled trial; the AIDS Clinical Trials Group is currently reviewing guidelines for such an investigation. In the absence of data from controlled studies, Dr. Klotman’s team has developed a mathematical model to estimate the influence of potent medication on the increase in the prevalence of end-stage renal disease (ESRD) among HIV-infected black patients. This model demonstrates that the decrease in prevalence of ESRD since 1995, which can be attributable to HIVAN, can be explained by the introduction of effective antiretroviral medication. The best fit of the available data indicates that effective therapy has reduced the growth in the number of HIV-infected black patients with ESRD by an estimated 28 percent. This model also predicts that the number of HIV-infected patients with end-stage renal disease (ESRD) will continue to rise in the absence of a drug with 100 percent efficacy and as the pool of patients at risk for developing HIVAN (i.e., black patients who are HIV-seropositive or living with AIDS) increases.
Dr. Klotman and colleagues’ recent experience with a patient who presented with ESRD during acute HIV seroconversion provides anecdotal evidence of a favorable effect of powerful antiretroviral therapy on very early onset HIVAN. Microcystic disease and focal segmental glomerulosclerosis were identified in a biopsy performed on a patient who was due to undergo dialysis, which are hallmarks of HIVAN. The patient was administered rigorous antiretroviral medication, and the plasma HIV RNA level was reduced below the detection threshold. The patient’s renal insufficiency was totally resolved after three months. Repeated biopsies revealed reversal of microcystic alterations observed prior to the initiation of strong antiretroviral medication and remission of collapsing glomerular disease with residual scarring. Collagen staining revealed persistent interstitial fibrosis, but the condition was significantly better than before therapy. Prior to powerful antiretroviral therapy, LTR circular viral DNA in biopsy samples was strongly positive, but this was no longer the case following treatment. In addition, the cure of renal failure was accompanied by a restoration of podocyte differentiation (as demonstrated by enhanced synaptopodin staining) and normal cell proliferation (as indicated by normal-ized Ki67 marker positivity). In situ hybridization for viral mRNA, however, revealed a (low) degree of ongoing viral transcription. Observations of this patient suggest that renal damage due to active infection may occur early in the course of HIV infection and that potent antiretroviral therapy may have a beneficial effect on the progression of HIVAN by halting such damage, thereby allowing for structural and functional reconstitution. In addition, the finding of ongoing viral transcription in the absence of active cellular infection during potent antiretroviral therapy supports the hypothesis that the kidney may be a viral reservoir; however, it is unknown whether the kidney may be a source of viral repopulation when the effectiveness of potent antiretroviral therapy in suppressing viral replication is reduced or when drug pressure is removed.