Fabry Disease is also known as Anderson-Fabry Disease. It was first discovered in 1898 by two scientists who were working independently. William Anderson, UK identified a systemic disorder in a 39-year-old man, and Johannes Fabry, Germany identified a similar condition in a 13-year-old boy. It wasn’t until the 1960s and 70’s though that the accumulation of Gb3 and the deficient enzyme α-Gal A were identified as the causal factors of this condition.
Contents
show
Key points of Fabry disease
- Multisystem lysosomal storage disorder involving skin, eye, kidney, heart, brain, and PNS.
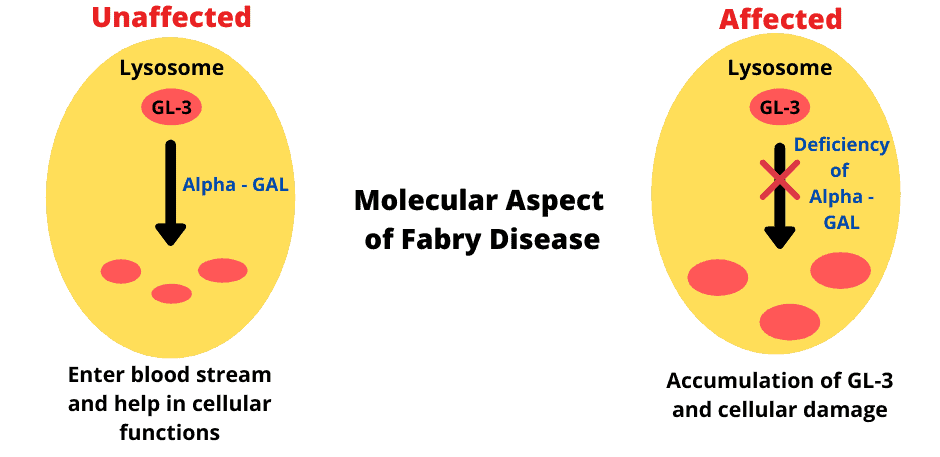
- Total lack or reduced activity of α-galactosidase due to mutations in GLA gene. >600 mutations have been identified and are listed in the online FD database.
- Accumulation of globotriaosylceramide (GL-3; also abbreviated Gb3)
- causing structural damage and cellular dysfunction, as well as triggering secondary, tissue-level responses, such as inflammation, ischemia, hypertrophy, and the development of fibrosis resulting in progressive organ dysfunction.
- X-linked disease.
- Affecting 1 in 40-60K males (can affect homozygous females)
- Onset is usually in childhood/ adolescence.
Clinical features
- Pain in the extremities (acroparesthesia)
- Cutaneous vascular lesions (angiokeratoma)
- Corneal opacities
- Tinnitus + hearing loss
- Proteinuria + progressive decline in renal function
Clinical features involving the central nervous system
- Small vessel disease
- Ischemic or hemorrhagic stroke in the young patient
- Microbleeds
- Subarachnoid hemorrhage
- Cerebral venous sinus thrombosis
- Transient global amnesia
- Dementia
- Depression
Treatment
Treatment can be Fabry specific and non-Fabry specific (supportive treatment).
Fabry specific
- Recombinant alpha-galactosidase A (alpha-Gal A). The FDA has approved only one replacement therapy in the U.S., called agalsidase beta (Fabrazyme).
- Migalastat (Galafold). This is a newer oral medication approved by the FDA in 2018. It’s slightly cheaper and more convenient than IVs. The drug helps the alpha-Gal A enzyme in your body work better.
Non-Fabry specific
Supportive treatment for
- Chronic kidney disease (CKD) – Renal replacement therapy.
- Cardiac disease – treatment for arrhythmia, heart attack, heart failure.
- Neurologic disease – treatment for stroke, dementia, depression.
- Gastrointestinal disease – treatment for abdominal pain, and indigestion.
- and treatment for other clinical manifestations
Monitoring for Fabry disease
- FBC
- Urinalysis
- ECG
- Echocardiogram
- Hearing test
- Brain imaging
Fabry disease and stroke
- Median age at first stroke for males is 39.0 Y.
- Median age at first stroke for females is 45.7 Y.
- Fifty percent of males and 38.3% of females experienced their first stroke before being diagnosed with Fabry disease.
- A total of 6.9% of males and 4.3% of females experienced stroke.
- Average life expectancy is 58 years.